A 1.0 Mol Sample of Which of the Following Compounds
20Atoms of Mg combine with atoms of F to form a compound. Percent by volume e n x 100 For example if 750 mL of white wine contains 80.

Sample Paper Science Quiz Chemistry Q 1 The Equivalent Weight
You have a one-mole sample of each of the following.
. The molecular weight of the compound is. M D 060. According to the information in the spectrum the atomic mass of the unknown element is closest to.
A 420 g sample of compound containing only C and H was analyzed. B Calculate the expected percentages of each in a sample of naturally occurring copper. A compound is analyzed and found to contain 121 carbon 162 oxygen and 717 chlorine by mass.
ML of ethanol the percent of ethanol by volume is L mL x 100 11 4. A compound contains 400 carbon 67 hydrogen and 533 oxygen by mass. G mol1 MgSO4 H2O 138.
THIS USER ASKED What is the number of moles of KF in a 29-gram sample of the compound. H 1 u. 10 mole potassium oxide d.
Which sample contains the greatest number of oxygen atoms. O 1 6 u Medium. A 020 mol sample of MgCl.
Molar masses are given after each formula. The results showed. Determine the molecular formula of napthalene from this information.
Calculate the empirical formula. 5 1 2 K m o l 1 k g T b H 2 O 1 0 0 C. The compound with molecules containing the greatest number of atoms of N and O has the greatest molar mass.
If 10 g samples of each compound were dehydrated which sample would lose the greatest mass of water. To determine its composition a sample is burned in excess oxygen producing the following results. Atoms of which of the following elements combine with.
А NO B NO NO2 с N20 D N205 А NO B NO NO2 с N20 D N205 This problem has been solved. Question 1099 These questions refer to the following compounds at 25 C. A Gas A CH4 16 gmol and gas B He 4 gmol b Gas A He 4 gmol and gas B CH4 16 gmol.
Nicotine has the formula C xH yN z. 10 mole sodium hydroxide b. 10 mole ammonium sulfate.
C 1 2 u. 2 4 C. View ChemQuestions98jpg from CHEM INORGANIC at Dartmouth College.
Calculate the molar mass of a sample if a single molecule weighs 534 10-23 g. Calculate the molecular mass of ethanoic acid C H 3 C O O H. N 2 O 5.
Y fraction of Cu-65. Mass-Volume 100 s of e e of Example. A CO b CO2 c HCO3 d H2C4O4.
A 113 1046 gmol B 120 gmol C 534 10-23 gmol D 322 gmol E none of these 10. The best explanation for the observation is that the molecules of the gas C. 10 mol 20 mol 050 mol 50 mol THIS IS THE BEST ANSWER explanation.
Are attracted to each other and do not exert as much force on the container walls as they would if they had no mutual attractions. Calculate the empirical formula of. A NO 2 B H 2 O C C 2 H 6 D NH 3 E CO 9.
X y 10 so x 10 -. A 10 L sample of a pure gas is found to have a lower pressure than that predicted by the ideal gas law. 10 mol of CO 2 070 mol of H 2O 020 mol of NO 2 Assume that all the atoms in nicotine are present as products.
10 mole calcium nitrate c. A 10 mol sample of which of the following compounds has the greatest mass. The mass spectrum for an unknown element is shown above.
A 10 mol sample of which of the following compounds has the greatest massA NOB NO2C N2OD N2O5 Get the answers you need now. Since each sample contains the same number of molecules 10mol of molecules the sample of N2O5N2O5 has the largest mass. An aqueous solution containing 288 gm of a non- volatile compound having the stoichiometric composition C x H 2 x O x in 90 gm water boils at 1 0 1.
A 10 mol sample of which of the following compounds has the greatest mass. X fraction of Cu-63. A 10 mol sample of which of the following compounds has the greatest mass.
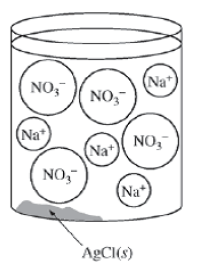
10 kg of seawater contains 35 g of salt. Sodium hydroxide ironIII sulfate ammonium nitrate barium carbonate leadIV oxide. 2 s and a 010 mol sample of KCls are dissolved in water and diluted to 500 mL.
Which of the following could be the identities of gas A and gas B. For which of the following compounds does 10 g represent 555 10-2 mol. If the molecular formula is C x H y O z K b H 2 O 0.
90 g NaCl in 10 L of solution. You have 10 mole of each compound below. 10 mole lithium phosphate e.
Under identical conditions it took 120 minutes for 10 L of gas B to effuse through the porous barrier. G mol1 FeSO4H2O 170. At 100 atmospheric pressure.
What is the concentration of Cl-in the solution. M C 050. Up to 24 cash back 19.
M B 030. Average atomic mass is 635 from periodic table. M E 10.
1 mole of a compound contains 1 mole of C and 2 moles of O. Which has the greatest mass. Assume that only these two isotopes exist.
Amazon Luna launches with freebies for Prime subscribersAmazon Luna special offer for Prime membersTry Amazon Luna Now. Percent by mass of salt in seawater 0 g 35 g 35 3.

Sch4u Exam Review Chemistry At Loyola

Printable Element List Of Chemistry Chart Elements Exercises Science Experiments Chemical Elements Chart 1 Element Chart Name Symbols Atomic Number

The Mole Atoms Molecules Quiz Quizizz
Solutions Conceptual Chemistry Quiz Quizizz

Quantitative Chemistry Ppt Video Online Download
Mefenamic Acid C15h15no2 Pubchem

Two Elements A And B Form Compounds Having Molecular Formula Ab 2 And Ab 4 When Dissolved In 20 G Of Benzene 1 Gof Ab 2 Lowers The Freezing Point By 2 3 K Whereas 1 0
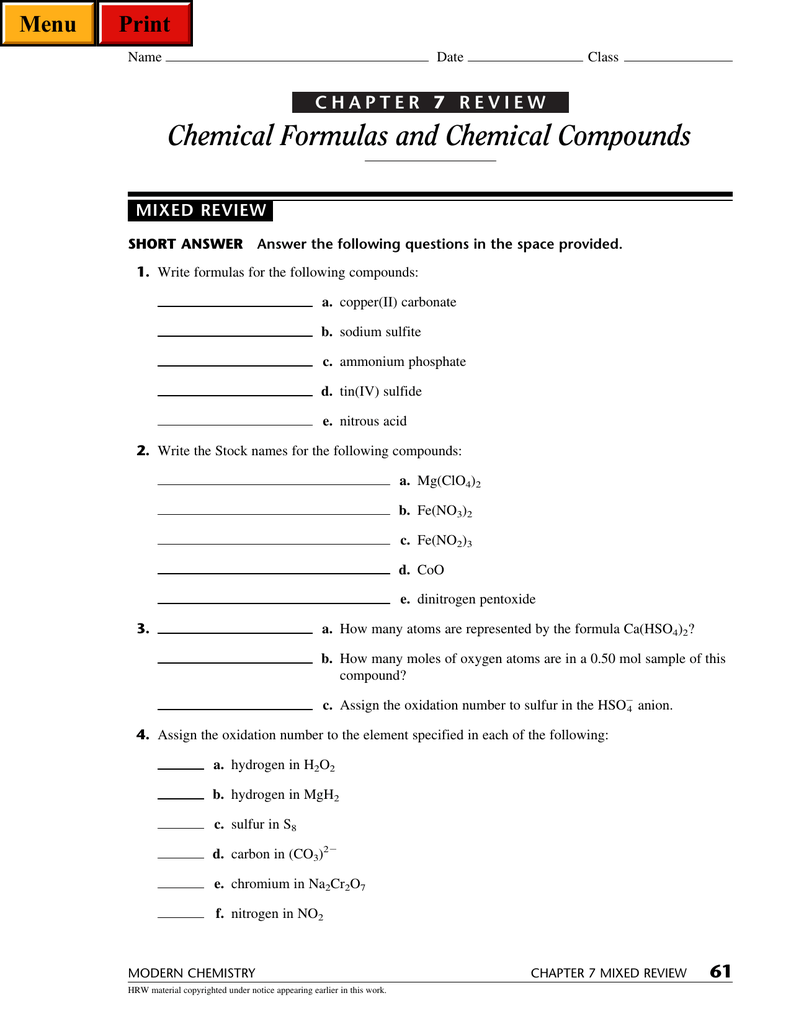
Chemical Formulas And Chemical Compounds Menu Print
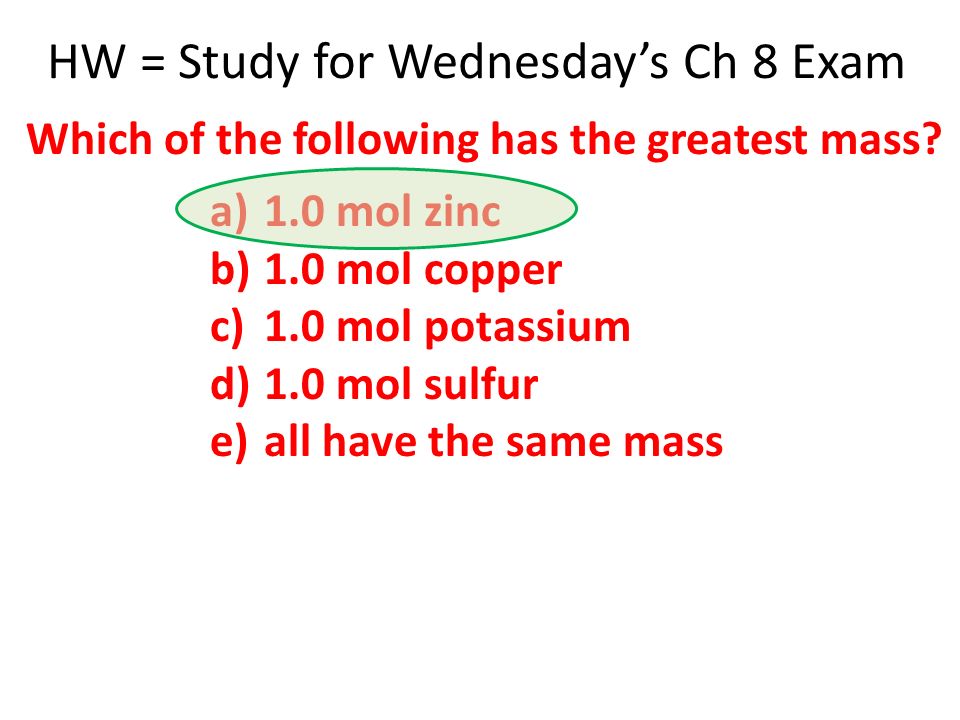
Hw Study For Wednesday S Ch 8 Exam A 1 0 Mol Zinc B 1 0 Mol Copper C 1 0 Mol Potassium D 1 0 Mol Sulfur E All Have The Same Mass Which Of The Following Ppt Download

Chemical Equations Chemistry Classroom Chemical Equation Chemical Science

Chemical Equations Chemistry Classroom Chemical Equation Chemical Science

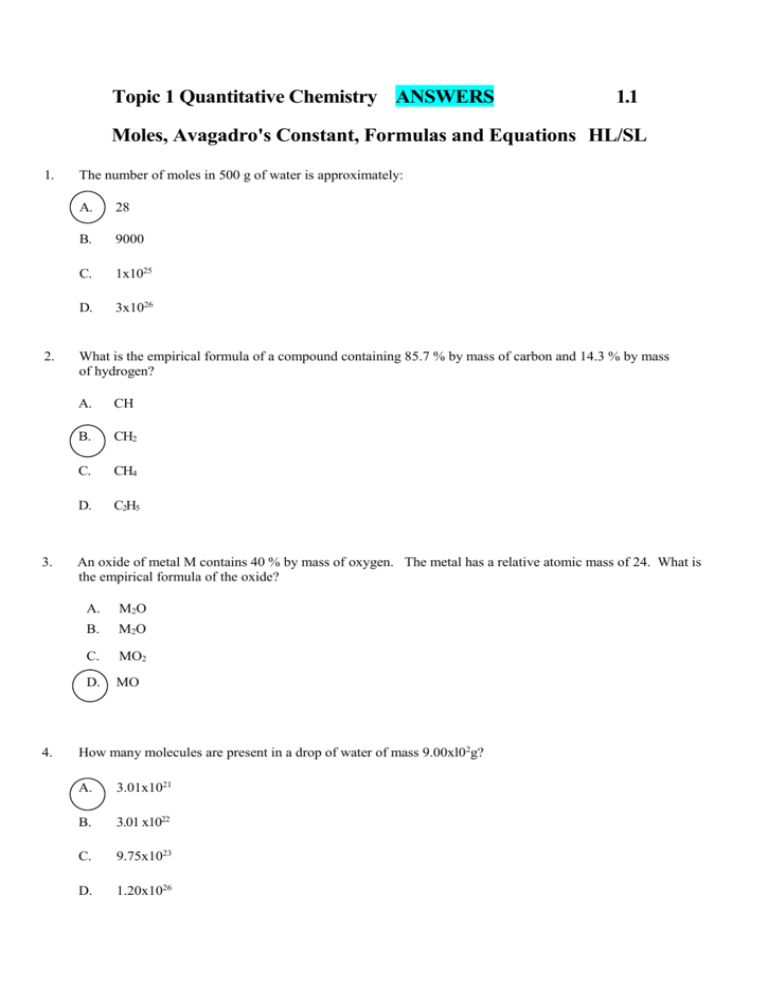
Topic 1 Quantitative Chemistry

Chemical Equations Chemistry Classroom Chemical Equation Chemical Science
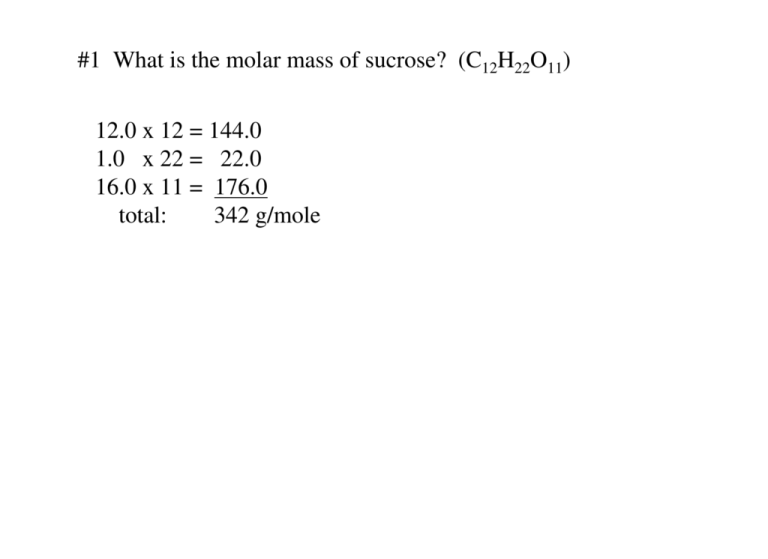
1 What Is The Molar Mass Of Sucrose C H O 12 0 X 12 144 0

Psi Ap Chemistry Aqueous Equilibria Part Ii Solubility Product

Ib Chemistry Sl Topic1 Questions And Answers 1 What Amount Of

Chemical Equations Chemistry Classroom Chemical Equation Chemical Science

Chemical Equations Chemistry Classroom Chemical Equation Chemical Science

Comments
Post a Comment